
 |
| Financial Review (Section 2 of 5) Pfizer Inc and Subsidiary Companies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
On This Page: | Total Revenues by Geographic Area | Changes in Geographic Total Revenues by Business Segment | Product Developments | | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Total Revenues by Geographic Area 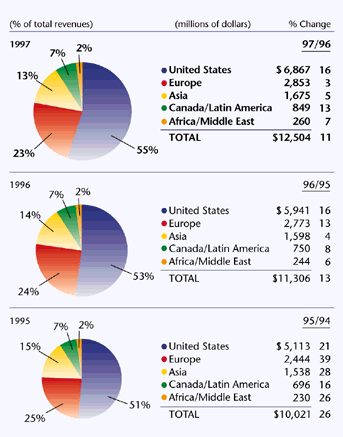 Changes in Geographic Total Revenues by Business Segment
Product Developments We continue to invest in R&D to provide future sources of revenue through the development of new products as well as additional uses for existing products. Following are certain significant regulatory actions by and filings pending with the U.S. Food and Drug Administration (FDA): 1997 FDA Approvals
Pending New Drug Applications (NDAs)
• Norvasc – Studies are being conducted to confirm the effectiveness of using Norvasc in the treatment of patients with congestive heart failure attributable to causes other than impaired blood flow to the heart. • Zithromax – We are conducting clinical programs to support a new use for Zithromax in decreasing cardiovascular risk in patients with atherosclerosis (a process in which fatty substances deposit within blood vessels) caused by a certain infection. • Zoloft – We are pursuing clinical programs to develop an oral liquid dosage form and to demonstrate Zoloft’s effectiveness in treating post-traumatic stress disorder (PTSD) as well as other disorders. Filings with the FDA for Zoloft oral liquid and the PTSD indication are expected during 1998. • Tikosyn (dofetilide) – for the treatment of a heart rhythm disorder — Regulatory filings are planned for the first quarter of 1998. • eletriptan – for the treatment of migraine headaches — Regulatory filings are planned for the third quarter of 1998. • droloxifene – for the prevention and treatment of osteoporosis, as well as for the prevention of breast cancer and the treatment of atherosclerosis • Alond (zopolrestat) – for the treatment of nervous system, kidney and cardiovascular disorders related to diabetes • voriconazole – for the treatment of fungal infections • darifenacin – for the treatment of irritable bowel syndrome and urinary urge incontinence | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||
| Advisory Information for Investors | ||||
| Copyright © 1997, 1998 Pfizer Inc All rights reserved. | ||||